Mitochondrial dynamics critically regulate cellular aging. Two-photon nonlinear structured illumination microscopy (TP-SIM), a low-phototoxicity live-cell imaging technique, was employed to dynamically track mitochondrial changes in senescent H9C2 cardiomyocytes. System validation in COS7 cells achieved 82-nm resolution, threefold higher than conventional microscopy, and sustained 5-min dynamic imaging. Compared to normal cells, senescent cells exhibited fragmented mitochondria. TP-SIM further captured impaired mitochondrial fusion dynamics during senescence through continuous imaging, demonstrating its dual capability for subcellular-resolution visualization and prolonged organelle tracking in live cells.
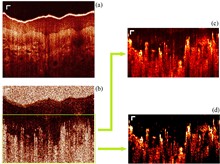
Tail artifacts are a significant issue in optical coherence tomography angiography (OCTA), as they cast shadows over underlying signals and interfere with the reconstruction of 3D vessel images. While many methods have been developed to reduce these artifacts, most only shorten the tails and fail to clearly distinguish between vessels and artifacts. In this Letter, we present an image processing technique designed to reduce artifacts. By combining structural images with OCTA images, we can more effectively distinguish between vessels and artifacts, leading to shorter and less pronounced tail artifacts. This method is integrated with other tail artifact removal techniques to further enhance image quality. The vessels of the palm are used as samples to experimentally demonstrate the effectiveness of our technique.
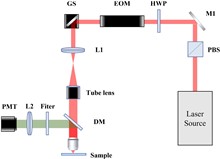
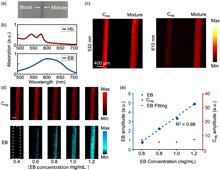
Vascular permeability (VP) plays a critical role in liver and kidney fibrosis progression. Traditional VP quantification methods use single-wavelength photoacoustic microscopy (PAM) with Evans Blue (EB) dye, which have limitations including signal attenuation and decreased accuracy. To overcome these issues, we developed a dual-wavelength PAM method with a spectral unmixing algorithm for quantitative VP evaluation in liver and kidney microvasculature. This approach allows for an accurate assessment of VP dynamics by analyzing hemoglobin and EB absorption. Using murine models of fibrosis, we found that fibrosis reduces vessel density and increases vessel diameter, providing valuable insights into VP changes during fibrosis progression.
An interventional fiber optic strategy, as a representative optical medical technology, is flexible, highly sensitive, minimally invasive, anti-electromagnetic, and has good biosafety. Fiber optics can approach deep cancer lesions and provide direct theranostics, which other optical technologies cannot achieve. However, the realization of cancer sensing and therapy relies on the functionalization of optical fibers, which requires the strict selection and optimization of functional materials used to modify the optical fibers, ensuring high photothermal conversion efficiency without affecting fluorescence detection efficiency. Herein, we propose the use of black phosphorus, which does not interfere with fluorescence and provides a safer and more efficient photothermal effect compared to other nanomaterials, such as graphene, graphene oxide, carbon nanotubes, and MXene. We propose a fiber-optic theranostic probe that combines nitroreductase (NTR) fluorescent molecules and a black phosphorus/gold nanostar (BP/AuNS) nanomaterial hydrogel to develop an integrated strategy for cancer sensing and photothermal therapy (PTT). The sensor has high sensitivity, and the limit of detection (LOD) is 1.61 ng mL-1. The BP/AuNS fibers have excellent photothermal effects, and the probe temperature reaches 212°C in air as 150 mW of pump power is delivered. In the phantom test, the simulation and test results showed that the fiber probe conferred hyperthermia (>45°C) to an area with a radius of 2.5 mm. These results indicate that the minimally invasive BP/AuNS fiber exhibits excellent sensing performance and high photothermal efficiency, making it promising for tumor diagnosis and treatment and potentially advancing the development of optical fiber medicine.
The quantification of microvascular blood flow velocity is pivotal in elucidating the characteristics of retinal microcirculation, and it plays a vital role in the early detection of numerous ophthalmic pathologies. However, non-invasive technology with a large field of view for directly measuring retinal capillary blood flow velocity is lacking. In this study, a novel imaging modality called encoding optical coherence tomography angiography (En-OCTA) is presented, utilizing retinal optical coherence tomography angiography (OCTA) encoding to accurately measure the absolute blood flow velocity in retinal capillaries. En-OCTA employs a scanning speed of 250 kHz to capture multiple OCTA images at two different locations on the same unbranched capillary. As red blood cells (RBCs) slowly flow through capillaries in a single file, intermittent light and dark changes can be observed on OCTA images. Analyzing the correlation of light and dark patterns in chronologically coded images of the capillary region allows for the determination of the lag time in RBC movement between two points. Combining this lag time with the distance between scan points allows the absolute blood flow velocity in the capillaries to be accurately calculated. Animal experiments demonstrate that the method can accurately measure capillary blood flow velocity and detect changes in velocity over the duration of anesthesia.
High-resolution label-free dual-mode full-field optical coherence tomography (FFOCT) is developed for simultaneous structural and functional imaging of mouse retinas, achieving both static contrasts gained from structural refractive index gradients and dynamic contrasts induced by endogenous cell motility. Imaging experiments on normal mouse retinas show that static FFOCT images better reveal the relative stationary structures like nerve fibers, vascular walls, and collagens, and dynamic FFOCT images show enhanced contrasts of cells with active intracellular metabolic motions, offering complementary information about major retinal layers. Specifically, dual-mode FFOCT imaging on early ischaemia/reperfusion (I/R) injured mouse retinas highlights the transparent ganglion cells at the cellular level without contrast agent labeling, visualizing their structural and dynamic alterations in early I/R injured retina.
Optical coherence elastography (OCE) can quantitatively obtain the viscoelasticity of tissues using rheological models and is widely applied to the clinical diagnosis of diseases. However, commonly used rheological models in OCE do not account for the distinctive dependence of high-frequency storage and loss moduli on frequency in tissues, which results in the rheological models failing to accurately measure the viscoelastic properties of tissues. In this paper, a modified Kelvin-Voigt fractional derivative model is presented based on the power-law behavior of soft tissues and the dependence of high-frequency complex shear modulus on frequency in living cells. In the rheometer and OCE tests, the modified model can provide the prediction of the power-law relationship between the low-frequency shear viscosity and frequency; compared with the Kelvin-Voigt and Kelvin-Voigt fractional derivative models, the modified model has a higher goodness-of-fit (accuracy >96%) for the high-frequency storage moduli of gelatin phantoms. Furthermore, the proposed model can reduce the root mean square error of fit by approximately 83% for the high-frequency (1–128 kHz) storage modulus of the polydimethylsiloxane phantoms obtained from publicly available data. Overall, the modified model accurately predicts the mechanical properties of biomimetic materials over a wide frequency range, with the potential to more accurately reflect pathological changes in tissues.
Wide-field second-harmonic generation (SHG) was used to obtain the second-harmonic signal from the entire image area for rapid imaging, despite the fact that conventional Gaussian beam illumination has low energy utilization efficiency, which makes it easy to overexpose the intensity of the image center area. However, flat-top beam illumination has uniform spatial distribution, thereby improving the photon excitation efficiency in the entire image region and reducing laser damage and thermal effect. By combining flat-top beam illumination and wide-field SHG polarization measurement, we can calculate more myosin fibril symmetrical axis orientations through polarization analysis of 16 images at a fast imaging speed while expanding the field of view. More importantly, the application of a flat-top beam can further improve the capability of polarization measurement in SHG microscopy.
Multi-focus parallel scanning can effectively increase laser fabrication throughput. However, the conventional approach of using a spatial light modulator (SLM) to generate multi-foci and scan this fixed number of foci with galvanometer scanners can only achieve a periodic scanning trajectory due to the low switching speed of the SLM. Here we demonstrate a multi-focus non-periodic scanning method for femtosecond lasers by using, instead, a fast-switching digital micromirror device (DMD) to generate a dynamic number of foci. The number of effective foci is quickly switched by introducing aberration to the undesired focus. In this way, the intensity allocated to each focus will not be affected by the number of foci, and a uniformity of 98% with different numbers of foci is achieved without adjusting the total laser energy. Finally, we validate the effectiveness of this scanning method by demonstrating corneal flap fabrication of porcine cornea in vitro.
Fluorescence lifetime imaging can reveal the high-resolution structure of various biophysical and chemical parameters in a microenvironment quantitatively. However, the depth of imaging is generally limited to hundreds of micrometers due to aberration and light scattering in biological tissues. This paper introduces an iterative multi-photon adaptive compensation technique (IMPACT) into a two-photon fluorescence lifetime microscopy system to successfully overcome aberrations and multiple scattering problems in deep tissues. It shows that 400 correction modes can be achieved within 5 min, which was mainly limited by the frame rate of a spatial light modulator. This system was used for high-resolution imaging of mice brain tissue and live zebrafish, further verifying its superior performance in imaging quality and photon accumulation speed.
We present a novel noncontact ultrasound (US) and photoacoustic imaging (PAI) system, overcoming the limitations of traditional coupling media. Using a long coherent length laser, we employ a homodyne free-space Mach–Zehnder setup with zero-crossing triggering, achieving a noise equivalent pressure of 703 Pa at 5 MHz and a -6 dB bandwidth of 1 to 8.54 MHz. We address the phase uncertainty inherent in the homodyne method. Scanning the noncontact US probe enables photoacoustic computed tomography (PACT). Phantom studies demonstrate imaging performance and system stability, underscoring the potential of our system for noncontact US sensing and PAI.
Wide-field linear structured illumination microscopy (LSIM) extends resolution beyond the diffraction limit by moving unresolvable high-frequency information into the passband of the microscopy in the form of moiré fringes. However, due to the diffraction limit, the spatial frequency of the structured illumination pattern cannot be larger than the microscopy cutoff frequency, which results in a twofold resolution improvement over wide-field microscopes. This Letter presents a novel approach in point-scanning LSIM, aimed at achieving higher-resolution improvement by combining stimulated emission depletion (STED) with point-scanning structured illumination microscopy (psSIM) (STED-psSIM). The according structured illumination pattern whose frequency exceeds the microscopy cutoff frequency is produced by scanning the focus of the sinusoidally modulated excitation beam of STED microscopy. The experimental results showed a 1.58-fold resolution improvement over conventional STED microscopy with the same depletion laser power.
We propose a laser speckle contrast imaging method based on uniting spatiotemporal Fourier transform. First, the raw speckle images are entirely transformed to the spatiotemporal frequency domain with a three-dimensional (3D) fast Fourier transform. Second, the dynamic and static speckle components are extracted by applying 3D low-pass and high-pass filtering in the spatiotemporal frequency domain and inverse 3D Fourier transform. Third, we calculate the time-averaged modulation depth with the average of both components to map the two-dimensional blood flow distribution. The experiments demonstrate that the proposed method could effectively improve computational efficiency and imaging quality.
Multiphoton microscopy is the enabling tool for biomedical research, but the aberrations of biological tissues have limited its imaging performance. Adaptive optics (AO) has been developed to partially overcome aberration to restore imaging performance. For indirect AO, algorithm is the key to its successful implementation. Here, based on the fact that indirect AO has an analogy to the black-box optimization problem, we successfully apply the covariance matrix adaptation evolution strategy (CMA-ES) used in the latter, to indirect AO in multiphoton microscopy (MPM). Compared with the traditional genetic algorithm (GA), our algorithm has a greater improvement in convergence speed and convergence accuracy, which provides the possibility of realizing real-time dynamic aberration correction for deep in vivo biological tissues.
We present for the first time, to the best of our knowledge, a needle probe for photoacoustic viscoelasticity (PAVE) measurements at a depth of 1 cm below the sample surface. The probe uses a gradient index rod lens, encased within a side-facing needle (0.7 mm outer diameter), to direct excitation light (532 nm) and detection light (1325 nm) focused on the sample, collecting and directing the returned detection light in a spectral domain low coherence interferometry system, which allows for obtaining optical phase differences due to photoacoustic oscillations. The feasibility of needle probe for PAVE depth characterization was investigated on gelatin phantoms and in vitro biological tissues. The experimental results in an in vivo animal model predict the great potential of this technique for in vivo tumor boundary detection.
Atherosclerotic cardio-cerebral vascular disease is the most common disease that threatens human health. Many researches indicated that oxidatively modified low-density lipoprotein (ox-LDL) is a key pathogenic factor of atherosclerosis. Here, we report the change of the secondary structure of ox-LDL caused by photoirradiation in an optofluidic resonator. The content ratios of amphipathic α-helices and β-sheets of ox-LDL are changed under laser beam illumination, resulting in an increasing binding rate of ox-LDL and ox-LDL antibodies. Our findings may provide a potential way for clinical atherosclerosis treatment and prompt recovery rate of atherosclerotic cardio-cerebral vascular disease by optical technology and immunotherapy.
Fluorescence microscopy technology uses fluorescent dyes to provide highly specific visualization of cell components, which plays an important role in understanding the subcellular structure. However, fluorescence microscopy has some limitations such as the risk of non-specific cross labeling in multi-labeled fluorescent staining and limited number of fluorescence labels due to spectral overlap. This paper proposes a deep learning-based fluorescence to fluorescence (Fluo-Fluo) translation method, which uses a conditional generative adversarial network to predict a fluorescence image from another fluorescence image and further realizes the multi-label fluorescent staining. The cell types used include human motor neurons, human breast cancer cells, rat cortical neurons, and rat cardiomyocytes. The effectiveness of the method is verified by successfully generating virtual fluorescence images highly similar to the true fluorescence images. This study shows that a deep neural network can implement Fluo-Fluo translation and describe the localization relationship between subcellular structures labeled with different fluorescent markers. The proposed Fluo-Fluo method can avoid non-specific cross labeling in multi-label fluorescence staining and is free from spectral overlaps. In theory, an unlimited number of fluorescence images can be predicted from a single fluorescence image to characterize cells.
Laser speckle imaging is a common technique to monitor blood flow. The fluctuations in speckle intensity can be related to the blood flow by calculating the speckle contrast, the ratio between the standard deviation of speckle fluctuations and the average intensity. However, this simple statistic calculation is easily affected by motion artifacts. In this study, we applied sample entropy analysis instead of calculating standard deviations of the speckle fluctuations. Similar to the traditional method, sample entropy-based speckle contrast increases linearly with flow rate but was shown to be more immune to sudden movements during an upper arm occlusion test.
In this paper, we propose and demonstrate a dual-beam delay-encoded Doppler spectral domain optical coherence tomography (OCT) system for in vivo measurement of absolute retinal blood velocity and flow with arbitrary orientation. The incident beam is split by a beam displacer into two probe beams of the single-spectrometer spectral domain OCT system with orthogonal polarization states and an optical path length delay. We validate our approach with a phantom and in vivo experiments of human retinal blood flow, respectively.
We proposed a nonlinear photoacoustic (PA) technique as a new imaging contrast mechanism for tissue thermal-nonlinearity characterization. When a sine-modulated Gaussian temperature field is introduced by a laser beam, in view of the temperature dependence of the thermal diffusivity, the nonlinear PA effect occurs, which leads to the production of second-harmonic PA (SHPA) signals. By extracting the fundamental frequency PA and SHPA signal amplitudes of samples through the lock-in technique, a parameter that only reflects nonlinear thermal-diffusivity characteristics of the sample then can be obtained. The feasibility of the technique for thermal-nonlinearity characterization has been studied on phantom samples. In vitro biological tissues have been studied by this method to demonstrate its medical imaging capability, prefiguring great potential of this new method in medical imaging applications.
Photoacoustic microscopy (PAM) has quickly developed into a noninvasive biomedical imaging technique to achieve detection, diagnosis, and monitoring. Compared with Q-switched neodymium-doped yttrium aluminum garnet or optical parametric oscillator lasers, a low-cost and small-size laser diode (LD) used as an alternative light source is conducive to achieving the miniaturization and integration for preclinical transformation. However, the LD’s low peak output power needs the high numerical aperture objective to attain tight focus, which limits the working distance (WD) of the system in only 2–3 mm, resulting in not achieving the backward coaxial confocal mode. Here, we present a compact visible LD-based PAM system with a reflective objective to achieve a 22 mm long WD and a 10 µm lateral resolution. Different depth subcutaneous microvascular networks in label-free mouse ears have successfully reappeared in vivo with a signal-to-noise ratio up to 14 dB by a confocal alignment. It will be a promising tool to develop into a handy tool for subcutaneous blood vessel imaging.
We present a deep learning approach for living cells mitosis classification based on label-free quantitative phase imaging with transport of intensity equation methods. In the approach, we applied a pretrained deep convolutional neural network using transfer learning for binary classification of mitosis and non-mitosis. As a validation, we demonstrated the performances of the network trained by phase images and intensity images, respectively. The convolutional neural network trained by phase images achieved an average accuracy of 98.9% on the validation data, which outperforms the average accuracy 89.6% obtained by the network trained by intensity images. We believe that the quantitative phase microscopy in combination with deep learning enables researchers to predict the mitotic status of living cells noninvasively and efficiently.
Photoacoustic imaging (PAI) with a handheld linear ultrasound (US) probe is widely used owing to its convenient and inherent dual modality capability. However, the limited length of the linear probe makes PAI suffer from the limited view. In this study, we present a simple method to substantially increase the view angle aided by two US reflectors. Both phantom and in vivo animal study results have demonstrated that the imaging quality can be greatly improved with the reflector without sacrificing the imaging speed.
Localized wavefront aberrations would introduce artifacts in biomedical imaging, which, however, are often neglected, as their compensations are at the cost of the field-of-view. Here, we show rarely reported local artifacts in two-photon imaging of dendrites beneath blood vessels in a mouse brain in vivo and interpret the phenomena via numerical simulations. The artifacts of divided parallel structures are found to be induced by coma and astigmatism, resulting from sample tilting and the cylinder shape of vasculatures, respectively. Different from that in single-photon microscopy, such artifacts in nonlinear microscopy show unique characteristics and should be recognized for proper interpretation of the images.
Microrobots-assisted drug delivery and surgery have been always in the spotlight and are highly anticipated to solve the challenges of cancer in situ treatment. These versatile small biomedical robots are expected to realize direct access to the tumor or disease site for precise treatment, which requires real-time and high-resolution in vivo tracking as feedback for the microrobots’ actuation and control. Among current biomedical imaging methods, photoacoustic imaging (PAI) is presenting its outstanding performances in the tracking of microrobots in the human body derived from its great advantages of excellent imaging resolution and contrast in deep tissue. In this review, we summarize the PAI techniques, imaging systems, and their biomedical applications in microrobots tracking in vitro and in vivo. From a robotic tracking perspective, we also provide some insight into the future of PAI technology in clinical applications.
In this study, the effects of purification, dehydration, and coagulation processes on the absorption and reduced scattering coefficients of chicken liver tissues have been investigated by using a single integrating sphere system. The purification process performed on the tissue samples to remove blood residue has been found to cause a slight change in the optical parameters. Although the dehydration process brings about an increase in the absorption coefficient due to the water loss, no direct relationship has been observed between the reduced scattering coefficient and the dehydration level of the tissue. In addition, it has been observed that there was a relatively small increase in the absorption coefficient and a significant increase in the reduced scattering coefficient after the coagulation process. Therefore, it can be said that the optical penetration depth decreased significantly after dehydration and coagulation processes unlike blood purification. Moreover, fluence rate distributions inside the fresh, blood purified, dehydrated, and coagulated tissue models have been investigated by using the Monte Carlo modeling of photon transport in multilayered tissues simulation code.
Currently, it is generally known that lens-free holographic microscopy, which has no imaging lens, can realize a large field-of-view imaging with a low-cost setup. However, in order to obtain colorful images, traditional lens-free holographic microscopy should utilize at least three quasi-chromatic light sources of discrete wavelengths, such as red LED, green LED, and blue LED. Here, we present a virtual colorization by deep learning methods to transfer a gray lens-free microscopy image into a colorful image. Through pairs of images, i.e., grayscale lens-free microscopy images under green LED at 550 nm illumination and colorful bright-field microscopy images, a generative adversarial network (GAN) is trained, and its effectiveness of virtual colorization is proved by applying it to hematoxylin and eosin stained pathological tissue samples imaging. Our computational virtual colorization method might strengthen the monochromatic illumination lens-free microscopy in medical pathology applications and label staining biomedical research.
This study investigated the feasibility of photoacoustic (PA) imaging of bone and characterization of bone features. By conducting the experiments on bovine femoral heads ex vivo, the light and ultrasonic penetration in bones was studied, together with the depth of PA imaging and measurement in bones. Then, the possibility of three-dimensional (3D) PA imaging of bones by raster scanning of the focusing transducer was studied. The micro-computerized tomography images of the bovine ribs with and without ethylenediaminetetraacetic acid (EDTA) treatment indicated that the 3D PA images could present the changes of bone microstructure resulting from the EDTA treatment. By using PA spectral analysis, the bone samples with and without the treatment of EDTA solution can be distinguished, and the microstructures can be characterized. This study was based on the bovine bone whose size is comparable to human bones, suggesting that PA technology can be used as a novel bone diagnostic technique.
We report on two strategies to design and implement the galvanometer-based laser-scanning mechanisms for the realization of reflectance confocal microscopy (RCM) and stimulated Raman scattering (SRS) microscopy systems. The RCM system uses a resonant galvanometer scanner driven by a home-built field-programmable gate array circuit with a novel dual-trigger mode and a home-built high-speed data acquisition card. The SRS system uses linear galvanometers with commercially available modules. We demonstrate video-rate high-resolution imaging at 11 frames per second of in vivo human skin with the RCM system and label-free biomolecular imaging of cancer cells with the SRS system. A comparison of the two strategies for controlling galvanometer scanners provides scientific and technical reference for future design and commercialization of various laser-scanning microscopes using galvanometers.
The recent development of stimulated Raman scattering (SRS) microscopy allows for highly sensitive biological imaging with molecular vibrational contrast, opening up a variety of applications including label-free imaging, metabolic imaging, and super-multiplex imaging. This paper introduces the principle of SRS microscopy and the methods of multicolor SRS imaging and describes an overview of biomedical applications.
Photoacoustic imaging has been developed to image the immune study at the macro scale. Macrophages play diverse roles in the acute response to infection and tissue repair. However, macrophages activities in acute inflammation at the microscopic level still remain challenging. In this work, we proposed optical-resolution photoacoustic microscopy to promptly monitor the labeled macrophages activities in normal and inflammatory groups. The result showed that many labeled macrophages emerged around the vessels firstly, then exuded into tissues, and finally disappeared in the inflammatory group injected with labeled macrophages. In summary, our method allows us to exactly image and track the immune cells of inflammatory diseases.